Phytase
Research by the Andlid group performed at Chalmers have in many projects focused on phytases produced by yeasts.
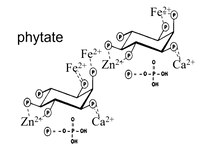
Phytases are enzymes degrading phytate (insositol hexaphoshpate; IP6), which is the main storage form of phosphorous in plant seeds. Especially cereals are very rich in phytate, and cereals are the number one staple food in the world.
Phytate binds minerals such as Fe, Zn and Ca in complexes not possible to degrade by the human digestive enzymes. The result is that the bound minerals become largely unavailabel for absorption in the human intestine. The same is true for pigs and poultry, where however the phosphorous is the key nutrient to release.
Phytate is not necessarily bad, but if mineral deficiency is a risk, it can be highly problematic. Iron and Zn deficiencies are common. Obviously this is very common in low income countries, but also for many population groups in the richer world, e.g women and elderly, or those eating a poor diet in general
In the Andlid Bio Solutions strain collection we have several high phytase yeast strains, some are so called wild-types (as formed by nature only, and found in nature, not in a lab), in our screenings found to be superior.
The figure shows phytate complexing minerals. The complexes are highly insoluble at most physiological pH levels, and the bound minerals are unavailable for intestinal absorption - unless a phytase is present (in the food, feed or intestina). That's where the yeast comes in.
The best wild-types were further improved by classical mutagenesis and selection (non GMO). Our strains are unique in production and release of phytase, and hence in capacity to degrade phytate
We also have precise knowledge on how to create high phytase yeasts of the species Saccharomyces cerevisiae (Baker’s yeast) by GMO technology.
Certain of our high phytase strains have shown to degrade in principal all phytate in foods such as bread. Degradation of phytate in the food releases iron and zinc, which leads to a improved availability for uptake in the human (or other monogastric animal's) intestine.
High phytase yeast strains may also be desired for other biotechnological productions, by releasing minerals for the producing microorganism itself, and hence enable a better utilization of the substrate. This decreases the need for mineral supplementation of a natural raw material used as culturing medium (these often contain phytate and often needs to be supplemented with minerals).
In addition to food, high phytase yeasts can be used in animal feed (especially poultry and pig) where in particular phosphorous availability and uptake is critical.
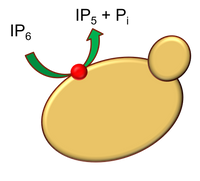
A schematic picture of yeast degrading phytate (IP6) in the surrounding medium. This happens by enzymatic hudrolysis of the phosphate groups, catalyzed by the yeast phytase (which is a special form of phosphatase). This leads to release of phosphate as well as bound iron and zinc (not shown in the fig).
Yeast phytase references (for complete list see google scholar)
Inositol hexaphosphate hydrolysis by Baker's yeast - Capacity, kinetics, and degradation products
M Türk, AS Sandberg, NG Carlsson, T Andlid (2000) Journal of Agricultural and Food Chemistry 48 (1), 100-104
Phytogenic and microbial phytases in human nutrition
AS Sandberg, T Andlid (2002) International journal of food science & technology 37 (7), 823-833
Metabolism of extracellular inositol hexaphosphate (phytate) by Saccharomyces cerevisiae
TA Andlid, J Veide, AS Sandberg (2004) International Journal of Food Microbiology 97 (2), 157-169
Degradation of phytate by high-phytase Saccharomyces cerevisiae strains during simulated gastrointestinal digestion.
AK Haraldsson, J Veide, T Andlid, ML Alminger, AS Sandberg (2005) Journal of agricultural and food chemistry 53 (13), 5438-5444
Secretion of non‐cell‐bound phytase by the yeast Pichia kudriavzevii TY13
A Hellström, L Qvirist, U Svanberg, J Veide Vilg, T Andlid (2015)
Journal of applied microbiology 118 (5), 1126-1136
Phytate degradation by microorganisms in synthetic media and pea flour
M Fredrikson, T Andlid, A Haikara, AS Sandberg (2002) Journal of Applied Microbiology 93 (2), 197-204
Phytate content is reduced and β‐glucanase activity suppressed in malted barley steeped with lactic acid at high temperature. AK Haraldsson, L Rimsten, ML Alminger, R Andersson, T Andlid, P Åman, ...
(2004) Journal of the Science of Food and Agriculture 84 (7), 653-662
Degradation of phytate by Pichia kudriavzevii TY13 and Hanseniaspora guilliermondii TY14 in Tanzanian togwa
AM Hellström, A Almgren, NG Carlsson, U Svanberg, TA Andlid (2012)
International journal of food microbiology 153 (1-2), 73-77
Assessing phytase activity–methods, definitions and pitfalls
L Qvirist, NG Carlsson, T Andlid (2015)
Journal of Biological Methods (JBM) 2 (1)
Improved extracellular phytase activity in Saccharomyces cerevisiae by modifications in the PHO system
J Veide, T Andlid (2006) International journal of food microbiology 108 (1), 60-67
Strain improvement of Pichia kudriavzevii TY13 for raised phytase production and reduced phosphate repression
L Qvirist, E Vorontsov, J Veide Vilg, T Andlid (2017) Microbial Biotechnology 10 (2), 341-353
Phytase active yeast (2007)
T Andlid, AS Sandberg, J Veide (2007) US Patent App. 11/693,771
Phytase active yeast (2007)
T Andlid, AS Sandberg, J Veide (2007) US Patent App. 11/693,759
Phytate degradation in composite wheat/cassava/sorghum bread: Effects of preincubation of Pichia kudriavzevii TY13 and presence of yeast extract
Serafina Lyidia, Svanberg Ulf, Andlid Thomas (2022) African Journal of Food Science 16 (12), 310-318
Degradation of Phytate in Composite Bread by addition of Phytase releasing Yeast Pichia kudriavzevii TY13
SL Vilanculos, U Svanberg, T Andlid (2020) J Nut Sci Heal Diet 1 (2), 30-37
Folate and phytase produced by yeasts
T Andlid, J Veide, S Hjortmo, A Hellström (2020)
Proceedings of the 23rd VH-Yeast Conference April 26-27, 2010 in Vienna
Phytase active yeast
T Andlid, AS Sandberg, J Veide (2008)
US Patent App. 11/693,767
Phytase production and Pi-repression of the Saccharomyces cerevisiae PHO system
J Veide, T Andlid (2003) YEAST 20, S222-S222
Multiple extracellular phytases secreted by Saccharomyces cerevisiae
J Veide, T Andlid (2001) YEAST 18, S302-S302